Arrange the atoms in order of increasing first ionization energy – The concept of first ionization energy, the energy required to remove an electron from an atom, plays a crucial role in understanding chemical reactivity and atomic structures. This article explores the factors influencing first ionization energy, the trends observed across the periodic table, and its diverse applications in various scientific fields.
Understanding these trends and applications enables chemists to predict chemical reactivity, design new materials, and delve into the intricacies of atomic physics and astrophysics.
Understanding First Ionization Energy
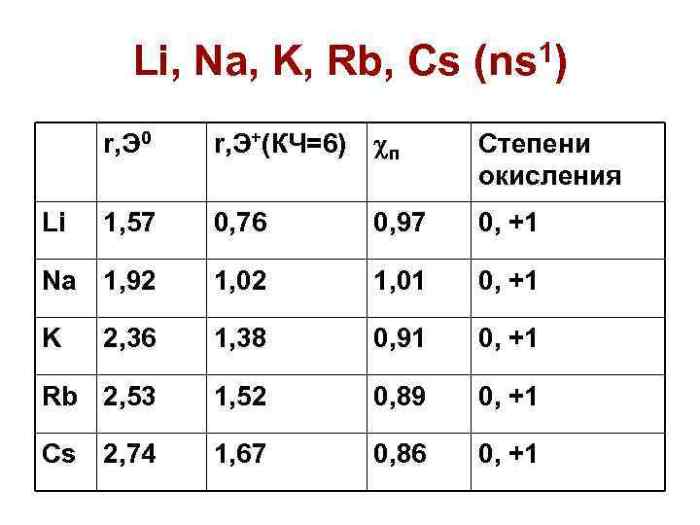
First ionization energy (IE) is the energy required to remove the most loosely bound electron from an atom in its gaseous state. It is a fundamental property of elements and plays a significant role in determining their chemical reactivity.
IE is affected by two main factors: atomic radius and nuclear charge. Larger atomic radii result in lower IE because the electron is further from the nucleus and experiences less electrostatic attraction. Conversely, higher nuclear charges lead to higher IE because the nucleus exerts a stronger pull on the electron.
Ordering Atoms Based on First Ionization Energy
| Atomic Number | Symbol | Element Name | First Ionization Energy (kJ/mol) |
|---|---|---|---|
| 1 | H | Hydrogen | 1312 |
| 2 | He | Helium | 2372 |
| 3 | Li | Lithium | 520 |
| 4 | Be | Beryllium | 900 |
| 5 | B | Boron | 801 |
Trends in First Ionization Energy Across the Periodic Table
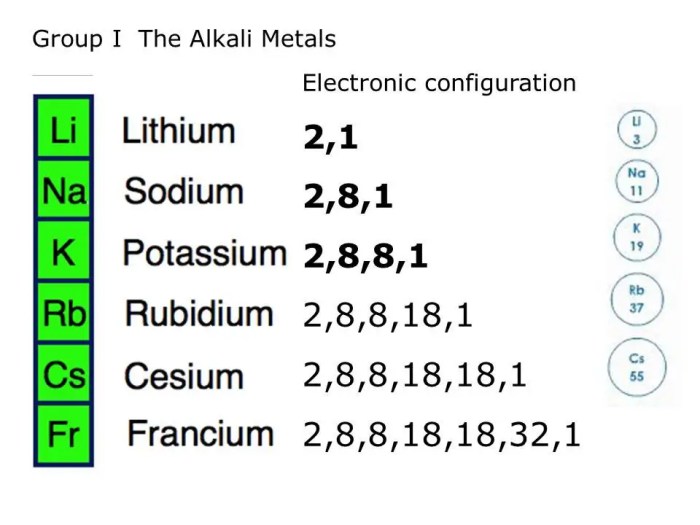
First ionization energy generally increases from left to right across a period and decreases from top to bottom within a group. This is because atomic radius decreases from left to right across a period due to increased nuclear charge, leading to higher IE.
Within a group, atomic radius increases from top to bottom, resulting in lower IE.
There are some exceptions to these trends. For example, IE of Be is higher than B, and IE of O is higher than N. These exceptions can be attributed to electronic configurations and specific atomic properties.
Applications of First Ionization Energy Data: Arrange The Atoms In Order Of Increasing First Ionization Energy
First ionization energy data has numerous applications in chemistry. It is used to:
- Predict chemical reactivity: Elements with lower IE are more reactive because they can lose electrons more easily.
- Understand atomic structures: IE provides insights into the electronic configurations and bonding behavior of atoms.
First ionization energy data also finds applications in materials science, astrophysics, and other fields.
Question & Answer Hub
What factors affect first ionization energy?
Atomic radius and nuclear charge are the primary factors influencing first ionization energy.
How does first ionization energy vary across the periodic table?
First ionization energy generally increases from left to right across periods and decreases from top to bottom within groups.
What are the applications of first ionization energy data?
First ionization energy data is used in predicting chemical reactivity, understanding atomic structures, designing new materials, and exploring astrophysical phenomena.