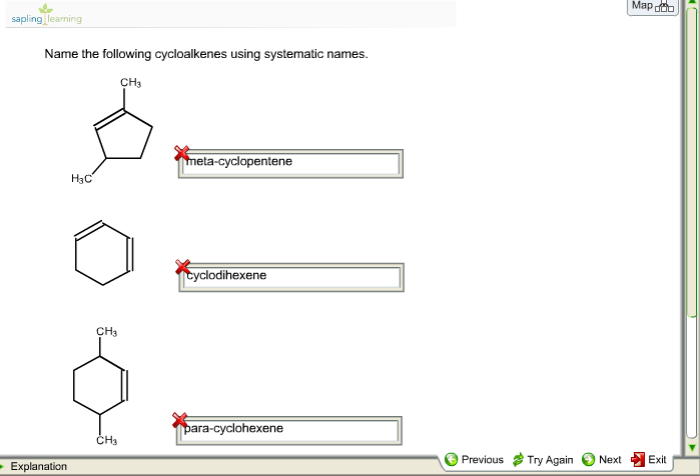
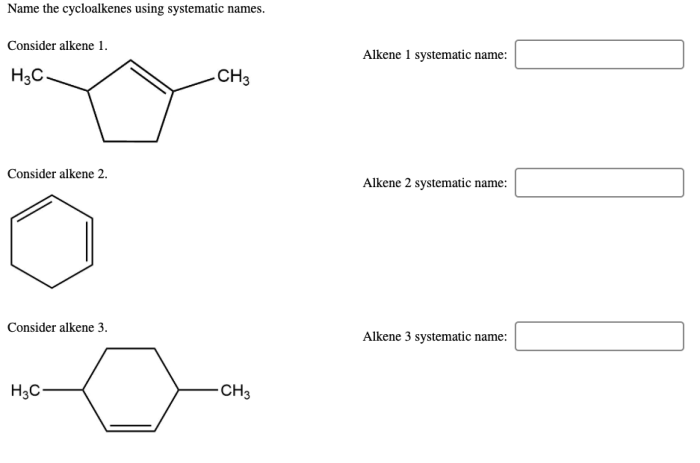
Name the cycloalkenes using systematic names – Introducing the nomenclature of cycloalkenes, a crucial aspect of organic chemistry, this comprehensive guide delves into the systematic naming of these cyclic hydrocarbons, providing a clear understanding of their structure and properties.
Delving into the intricacies of cycloalkene nomenclature, we will explore the IUPAC rules, identify structural characteristics, and uncover the significance of using systematic names in scientific communication.
Cycloalkene Nomenclature Basics
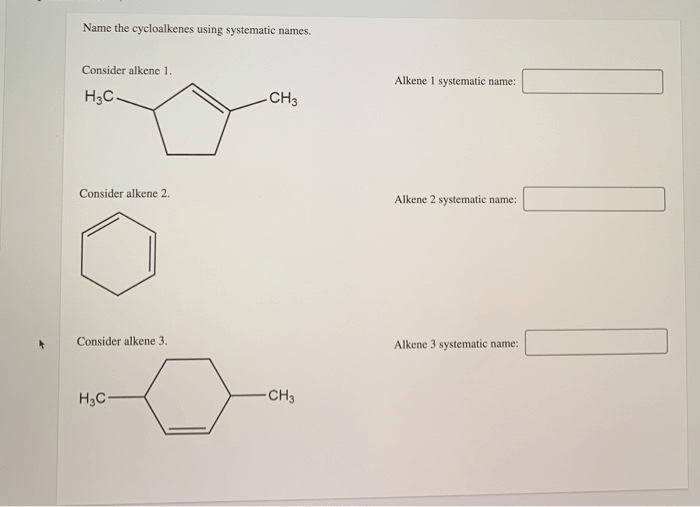
Cycloalkenes are cyclic hydrocarbons that contain one or more double bonds. The IUPAC rules for naming cycloalkenes are as follows:
- The base name of the cycloalkene is derived from the number of carbon atoms in the ring.
- The suffix “-ene” is added to the base name to indicate the presence of a double bond.
- The location of the double bond is indicated by a number following the base name.
For example, the cycloalkene with four carbon atoms and one double bond is named cyclobutene. The cycloalkene with six carbon atoms and one double bond is named cyclohexene.
It is important to use systematic names for cycloalkenes because it allows chemists to communicate clearly and unambiguously about these compounds.
Identifying Cycloalkenes
Cycloalkenes have the following structural characteristics:
- They contain one or more double bonds.
- The double bonds are located within a ring of carbon atoms.
- The carbon atoms in the ring are all sp 2hybridized.
Cycloalkenes can be distinguished from other types of hydrocarbons by their IR spectra. Cycloalkenes have a characteristic absorption band at around 1650 cm -1, which is due to the C=C double bond.
Common Cycloalkenes, Name the cycloalkenes using systematic names
Some of the most common cycloalkenes encountered in chemistry include:
- Cyclobutene
- Cyclopentene
- Cyclohexene
- Cycloheptene
- Cyclooctene
These cycloalkenes are all colorless liquids with boiling points that increase with increasing ring size. They are all relatively reactive and can undergo a variety of reactions, including addition, cycloaddition, and polymerization.
Cycloalkene Reactivity
Cycloalkenes undergo a variety of reactions, including:
- Addition reactions:Cycloalkenes can react with a variety of reagents to add two atoms or groups of atoms to the double bond. These reactions include hydrogenation, halogenation, and hydration.
- Cycloaddition reactions:Cycloalkenes can react with other unsaturated compounds to form cyclic products. These reactions include the Diels-Alder reaction and the ene reaction.
- Polymerization reactions:Cycloalkenes can polymerize to form a variety of polymers. These polymers include polyethylene, polypropylene, and polystyrene.
The reactivity of cycloalkenes is influenced by a number of factors, including the ring size, the number of double bonds, and the presence of substituents.
Cycloalkene Synthesis
There are a number of different methods that can be used to synthesize cycloalkenes. These methods include:
- Ring-closing metathesis:This method involves the reaction of a diene with a transition metal catalyst to form a cycloalkene.
- Cyclopropanation:This method involves the reaction of an alkene with a carbene to form a cyclopropane. Cyclopropanes can then be converted to other cycloalkenes by a variety of methods.
- Cyclodehydration:This method involves the removal of water from a diol to form a cycloalkene.
The choice of synthesis method depends on the desired cycloalkene and the starting materials that are available.
Cycloalkene Applications
Cycloalkenes are used in a variety of applications, including:
- Pharmaceuticals:Cycloalkenes are used in the synthesis of a variety of pharmaceuticals, including antibiotics, anti-inflammatory drugs, and anti-cancer drugs.
- Materials science:Cycloalkenes are used in the synthesis of a variety of materials, including plastics, polymers, and composites.
- Food chemistry:Cycloalkenes are used in the synthesis of a variety of food additives, including flavors, fragrances, and colorants.
Cycloalkenes are an important class of organic compounds with a wide range of applications. The understanding of their chemistry is essential for the development of new and improved products and technologies.
Q&A: Name The Cycloalkenes Using Systematic Names
What are the key principles of cycloalkene nomenclature?
The key principles involve identifying the parent chain, numbering the ring, and incorporating prefixes to indicate substituents and double bonds.
How does systematic naming aid in the identification of cycloalkenes?
Systematic names provide a precise and unambiguous way to identify cycloalkenes, facilitating communication among scientists and ensuring accurate documentation.